Alkene metathesis is widely utilized in synthetic chemistry and materials science. Despite the presence of latent alkene subunits in aromatic compounds, metathesis reactions involving the carbon–carbon bonds of aromatic rings remain an unexplored area. This limitation is primarily due to thermodynamic constraints and the lack of suitable reaction pathways.Developing ring-opening reactions of aromatic compounds is a pressing and highly attractive challenge. The team led by Prof. Wen-Xiong Zhang at the College of Chemistry and Molecular Engineering, Peking University, has reported the ring-opening metathesis of benzene promoted by rare-earth metallacycles. The research results were recently published online in Journal of the American Chemical Society on December 12, 2024 (J. Am. Chem. Soc. 2025, 147, 1300) with the title “Rare-Earth Metal-Enabled Ring-Opening Metathesis of Benzene”.
Given the huge thermodynamic limitation, a seemingly feasible approach involves photoexcitation of aromatic compounds to the triplet state, enabling reactions with alkenes. However, the [2+2] cycloaddition intermediate, bicyclo [4.2.0] octadiene, generated in this process tends to undergo 6π retro-electrocyclization to form cyclooctatriene rather than yielding metathesis products via [2+2] cycloreversion(Fig. 1a).In this context, the researchers proposed a hypothesis using a carbodianion as a substitute for alkenes in aromatic ring-opening metathesis. This hypothesis is founded on two factors: first, the introduction of a carbodianion enhances reactivity while providing additional thermodynamic driving force through stabilization of the anionic species; second, the presence of negative charges in the [2+2] cycloaddition intermediate suppresses the typical 6π retro-electrocyclization pathway, thereby facilitating [2+2] cycloreversion to afford metathesis products (Fig. 1b).
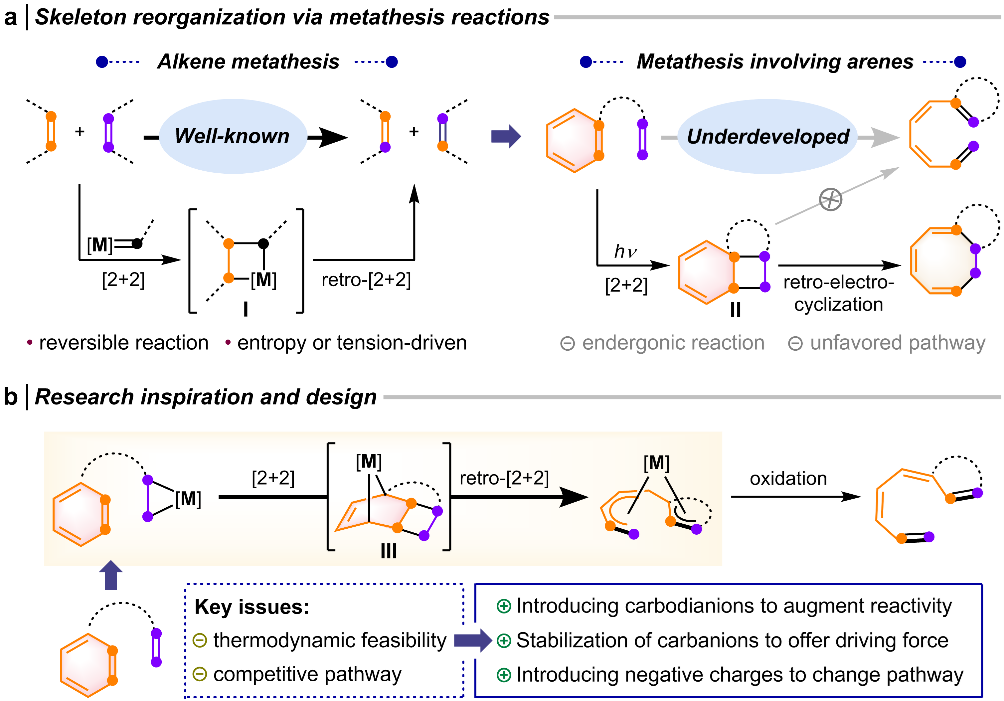
Fig. 1 Research strategy for exploring the ring-opening metathesis of aromatic compounds.
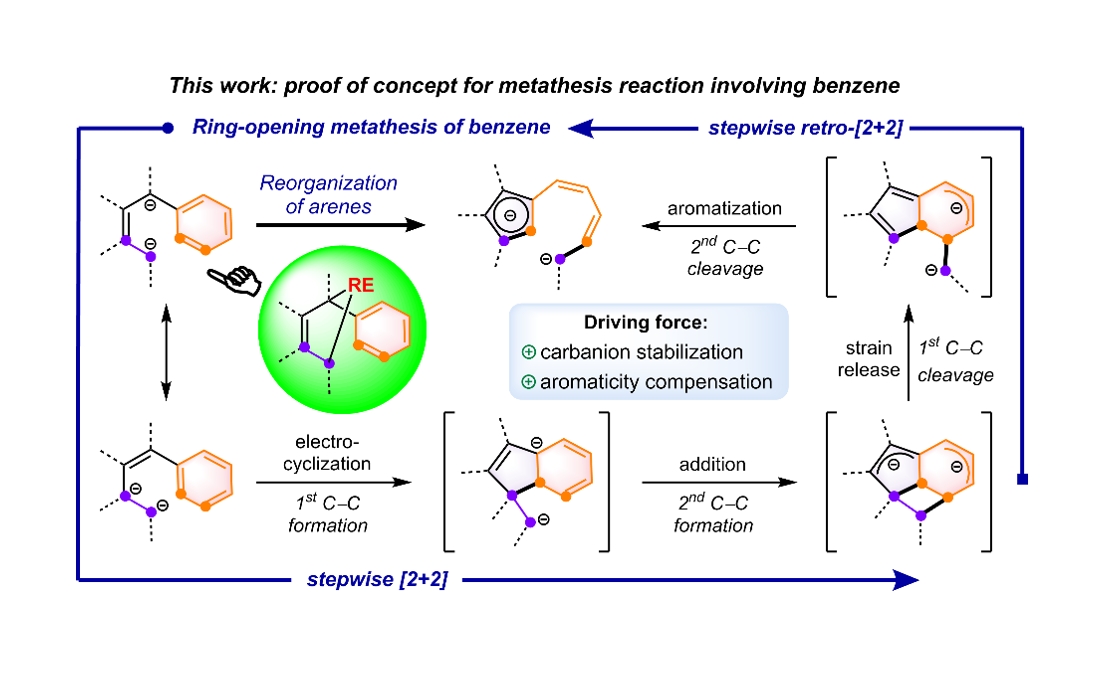
In recent years, Prof. Wen-Xiong Zhang's group has made significant advancements in the field of rare-earthmetallacyclic chemistry (J. Am. Chem. Soc. 2024, 146, 15609; J. Am. Chem. Soc. 2023, 145, 6633; Cell Rep. Phys. Sci.2022, 3, 100831; J. Am. Chem. Soc. 2021, 143, 9151; J. Am. Chem. Soc. 2020, 142, 10705; J. Am. Chem. Soc. 2019, 141, 20547). In 2023, they reported a rare-earth-mediated cross-carbanion coupling reaction, which successfully yielded rare-earth metallacyclopentene species (Cell Rep. Phys. Sci. 2023, 4, 101479). Leveraging the high reactivity and synergistic effects of the carbodianions within rare-earth metallacyclopentenes, they discovered the first ring-opening metathesis reaction of benzene, thereby confirming their proposed strategy (Fig. 2).
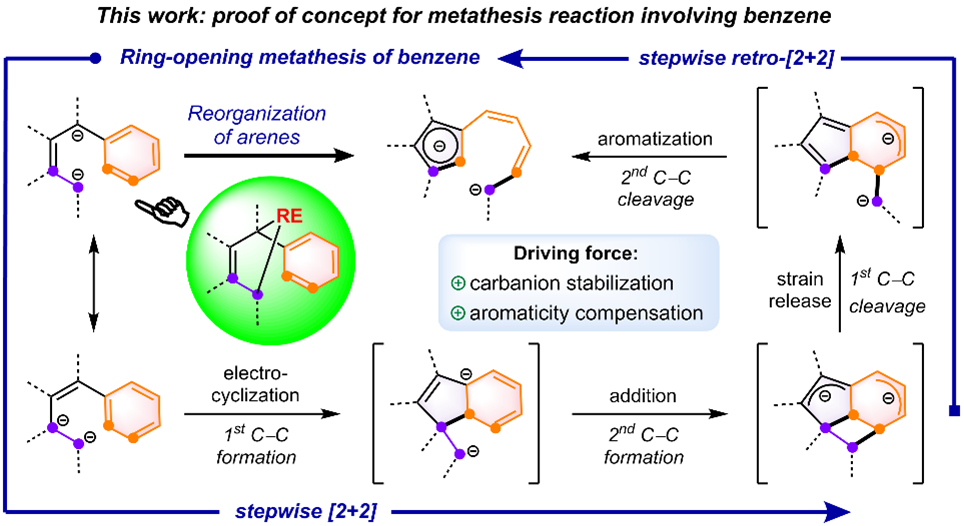
Fig. 2 Realization of the ring-opening metathesis reaction of benzene.
Firstly, they discovered that lutetacyclopentene 1a undergoes thermal transformation to compound 2a (Fig. 3a). The single-crystal structure of 2a reveals it as a half-sandwich complex containing a multi-substituted cyclopentadienyl (Cp) ligand with a coordinative side arm. The Cp framework is connected to the coordinated C11 atom via a spiro-diene fragment (Fig. 3b).
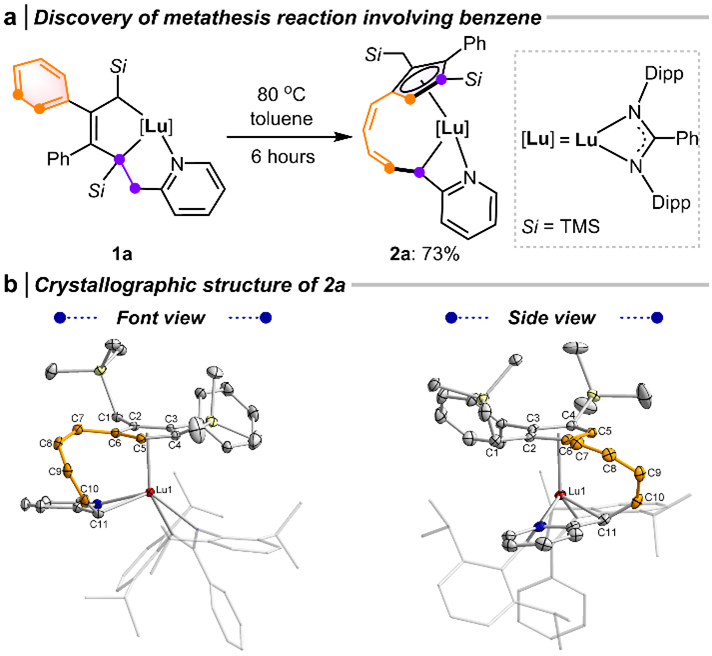
Fig. 3 Reaction discovery and crystal structure of the product.
Subsequently, the reaction mechanism was thoroughly investigated. Adding a tBuNC ligand to the reaction system enabled the capture of intermediate 3, formed prior to the metathesis step (Fig. 4a). The conversion of 1a to 3 involves a formal 1,5-H shift. Deuterium-labeling experiments confirmed hydrogen migration from C11 to C1, validating 3 as the reaction intermediate. Isotope-labeling studies revealed that the cleavage of the C4–C11 single bond and the C5–C10 bond within the benzene ring was accompanied by the formation of two new bonds: C4–C5 and C10–C11 (Figures 4b, c). These findings provide preliminary insights into the reaction pathway: compound 1 first undergoes a 1,5-H shift to yield intermediate 3, followed by benzene ring-opening metathesis to afford 2 (Fig. 4d).
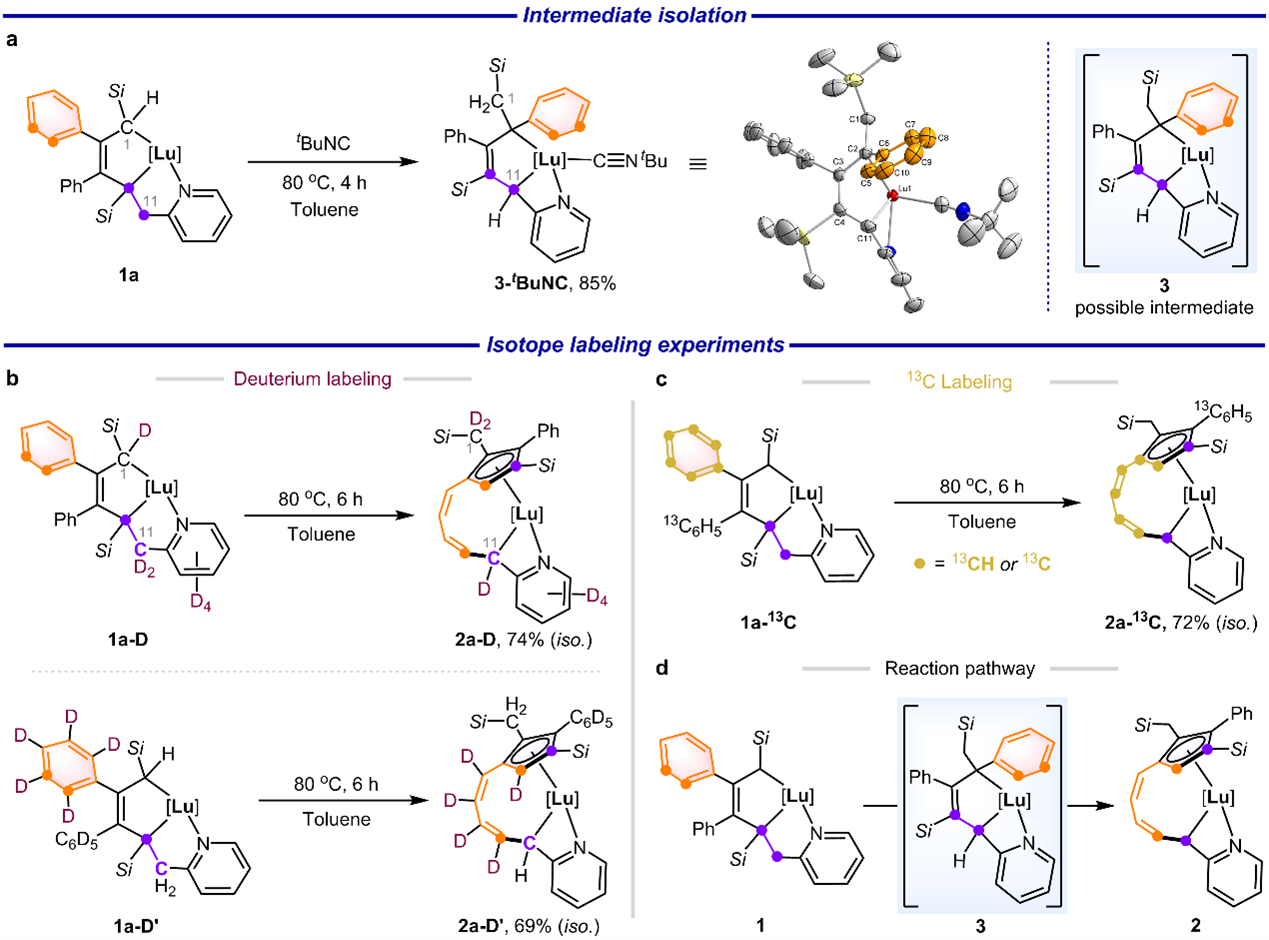
Fig. 4 Mechanistic investigation.
Mechanistic calculations by Prof. Gen Luo's group at Anhui University revealed the key steps in the benzene ring-opening metathesis reaction. The process begins with a 1,5-H shift to form intermediate 3. Intermediate 3 undergoes ring contraction to produce intermediate B, which subsequently undergoes a 1,5-electrocyclization to form intermediate C. This step establishes the C4–C5 bond while disrupting the aromatic system. The Lu–C bond then attacks the C="C" double bond to form the C10–C11 bond, generating the strained intermediate D. Driven by ring strain release, the C4–C11 bond cleaves to yield intermediate E. Finally, the original C5–C10 bond of the benzene ring breaks under aromatization to form intermediate F, which isomerizes to the final product 2a. The overall metathesis proceeds via a stepwise [2+2] cycloaddition to generate a strained four-membered intermediate, followed by a stepwise [2+2] cycloreversion, leading to the reconstruction of the benzene framework.
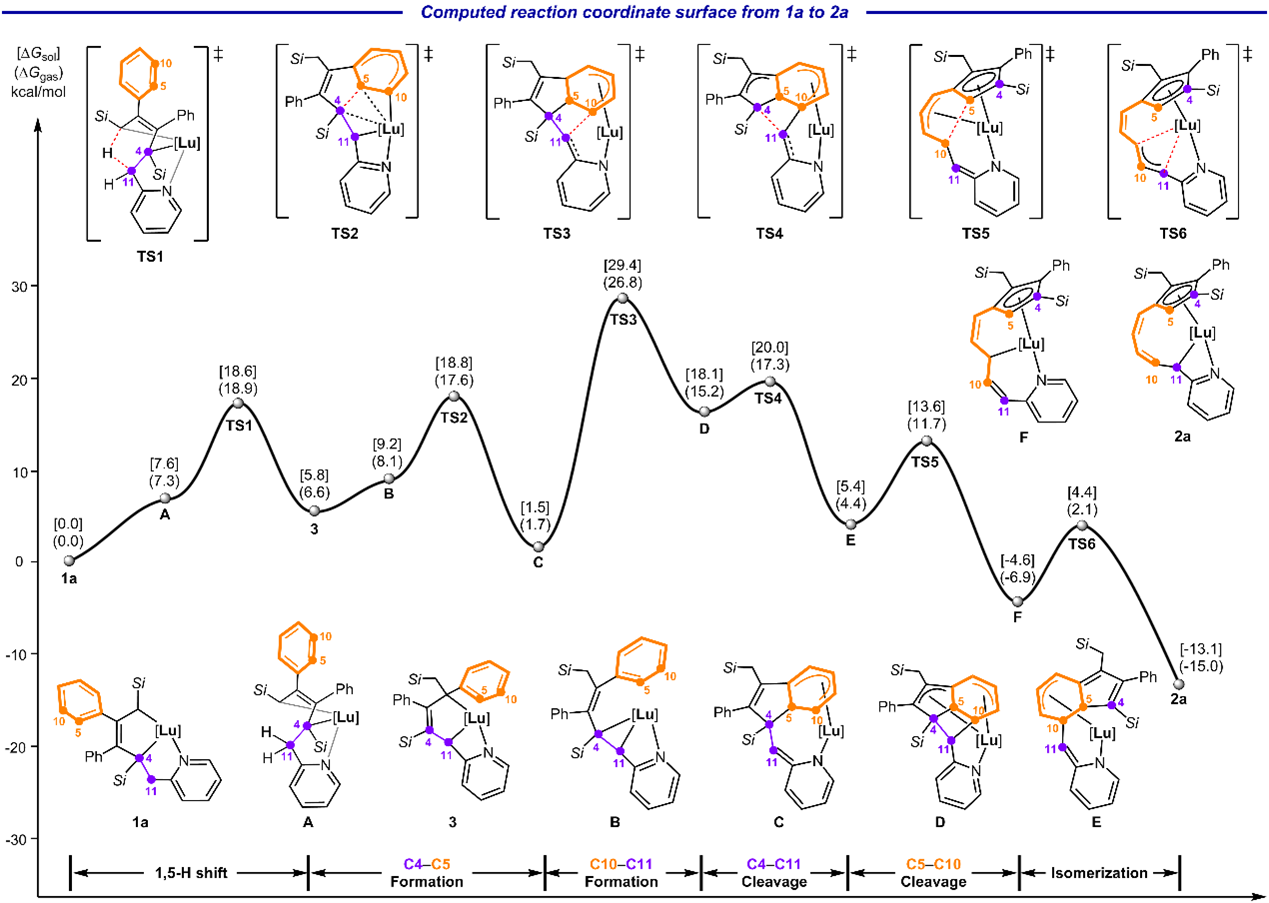
Fig. 5 Computed reaction coordinate surface.
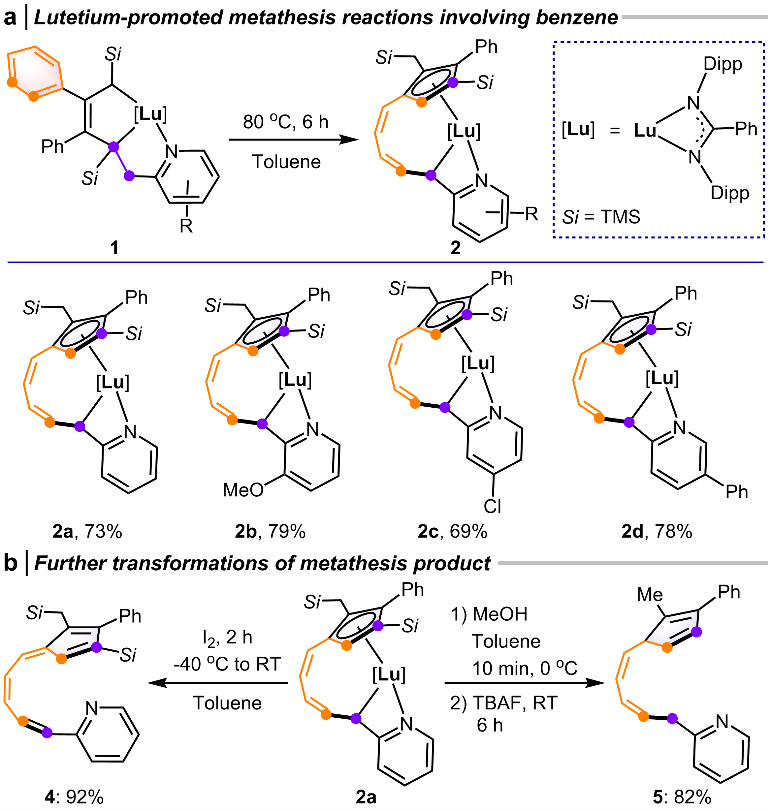
Fig. 6 Reaction scope expansion and further transformations of metathesis products
The researchers further extended the scope of the benzene ring-opening metathesis reaction. Lutetacyclopentenes with various substituents were successfully converted into the target products with high yields (Fig. 6a). Oxidation of 2a yielded fulvene-type compound 4, which can be regarded as a formal metathesis product of benzene and an alkene (Fig. 6b). Additionally, protonolysis and desilylation of 2a afforded multi-substituted cyclopentadiene compound 5 (Fig. 6b). These transformations highlight the synthetic versatility and potential applications of benzene ring-opening metathesis reactions.
In summary, leveraging the high reactivity and synergistic effect of rare-earth metallacycles, the benzene ring-opening metathesis reactions were successfully realized. This work uncovers a novel activation mode of aromatic compounds, expands the scope of organic metathesis reactions, and provides a new strategy for activating inert carbon–carbon bonds. Dr. Wei Liu is the first author of the study, with Prof. Wen-Xiong Zhang and Prof. Luo Gen as corresponding authors. This work was supported by the National Key R&D Program of China (no. 2021YFF0701600), National Natural Science Foundation of China (nos. 22371006, 22131001, 22271001) and Beijing National Laboratory for Molecular Sciences (BNLMS-CXXM-202401).
Original link for the paper: https://doi.org/10.1021/jacs.4c15650