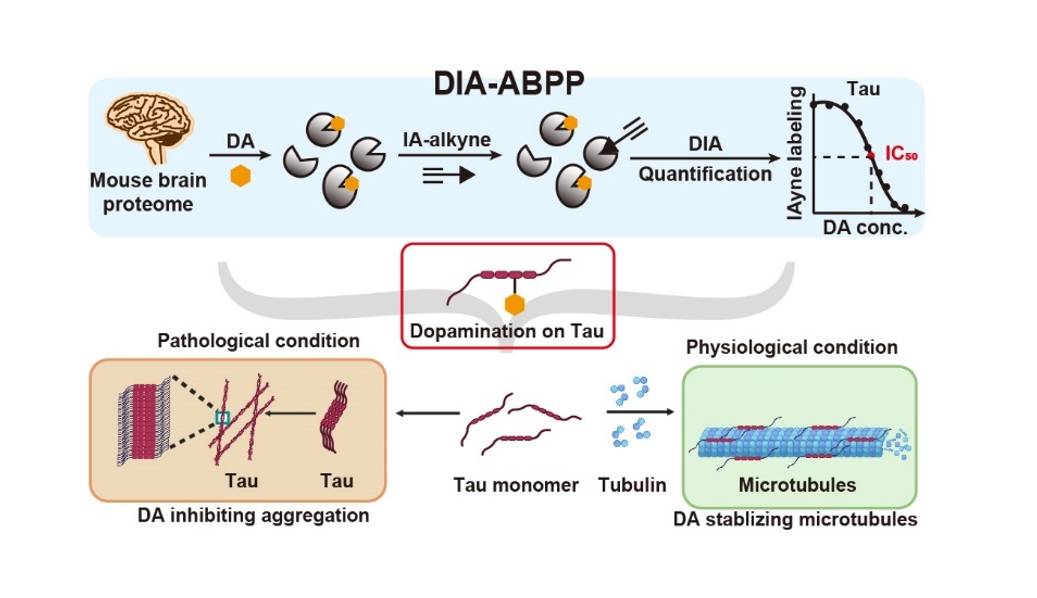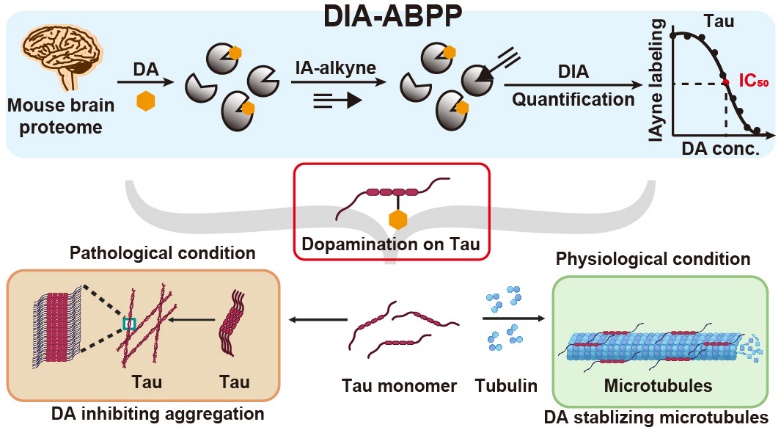
Dopamine (DA) is one of the most important neurotransmitters, functionally involved inthe regulation of nervous activity.In the central nervous system, the synthesis, transport, and release of DA are strictly controlled. This is because the catecholamine structure of DA can be easily oxidized to produce a series of electrophilic products, which can react with nucleophilic residues on proteins through Michael addition, forming quinone modifications (termed ‘dopamination’ in this work). However, our understanding of DA functionality remains incomplete.
On February 20th, 2025, the research group led by Prof. Chu Wang from the College of Chemistry and Molecular Engineering at Peking University published a research article entitled “Quantitative Chemoproteomics Reveals Dopamine’s Protective Modification of Tau”in Nature Chemical Biology (DOI: 10.1038/s41589-025-01849-9). In this paper, the authors developed a quantitative chemoproteomic strategy to site-specifically measure proteins’ dopamination globally.Among their findings, they discovered a protective role of DA in regulating the function of microtubule-associated protein Tau, which enhances our understanding of the physiological and pathological functions of DA in the human brain.
The authors first developed a quantitative chemoproteomic strategy, DIA-ABPP, to measure dopamination. Considering that dopamination occurs predominantly on cysteines, chemoproteomic technologies for cysteinome profiling were readily applicable for globally quantifying dopamination in a site-specific manner. The authors pretreated proteome samples with a series of concentrations of DA and labeled the proteome samples with IA-alkyne. Using a regular chemoproteomic workflow, the acquired IA-alkyne-labeled peptides were analyzed by LC–MS/MS with DIA quantification. More than 6,000 dopamination sites were quantified, and half-maximal inhibitory concentration values for 63 hypersensitive sites were measured. Among these, hypersensitive dopamination of two cysteines in microtubule-associated protein Tau was biochemically validated and functionally characterized to prevent Tau’s amyloid fibrillation and promote Tau-mediated assembly of microtubules. Additionally, endogenous dopamination of Tau in mouse brain was detected through targeted mass spectrometry analysis.
In summary, a quantitative chemoproteomic platform was developed to identify and quantify dopamination sites in the mouse brain. The highly conserved cysteines of Tau were proven to be dopaminated, and this modification prevented Tau’s amyloid fibrillation and promoted Tau-mediated assembly of microtubules.This study not only provides a global portrait of dopamination but also discovers a protective role of DA in regulating the function of Tau, enhancing our understanding of the physiological and pathological functions of DA in the human brain. It also holds significant guiding importance for the development of drugs targeting Tau.
The corresponding authors of this work are Prof. Chu Wang, Prof. Cong Liu (Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences) and Dr. Weidi Xiao (Peking University Chengdu Academy for Advanced Interdisciplinary Biotechnologies).The first authors are Dr. Qianwen Wang from Chu Wang’s lab, Dr. Zhengtao Liu from Cong Liu’s lab, and Ms. Youjia Wang from Chu Wang’s lab. This work was supported by the National Natural Science Foundation of China, National Key Research and Development Projects, and Beijing National Laboratory for Molecular Sciences.
Link of the paper: https://doi.org/10.1038/s41589-025-01849-9