Although immunotherapy has revolutionized cancer treatment, its efficacy is affected by multiple factors, particularly those derived from the complexity and heterogeneity of the tumor-immune microenvironment (TIME). Multiple immune subsets in TIME can work in combination to promote or impede the efficacies of immunotherapies. Strategies that simultaneously and synergistically engage multiple immune cells in TIME remain highly desirable but challenging.
On November 5th, 2024, the research group led by Prof. Peng R. Chen from the College of Chemistry and Molecular Engineering at Peking Universitypublished a research article entitled “Multimodal targeting chimeras enable integrated immunotherapy leveraging tumor-immune microenvironment” in Cell (DOI: 10.1016/j.cell.2024.10.016). In this paper, the authors reported a triple orthogonal linker (T-Linker)-basedplatform that enabled the programmable integration of multiple therapeutic modules into single agents. The resulting multimodaltargeting chimeras (Multi-TACs) were capable of co-engagingmultiple immune cells on targeted tumors in TIME. These studies have provide new modalities and open avenues for immunotherapy and beyond.
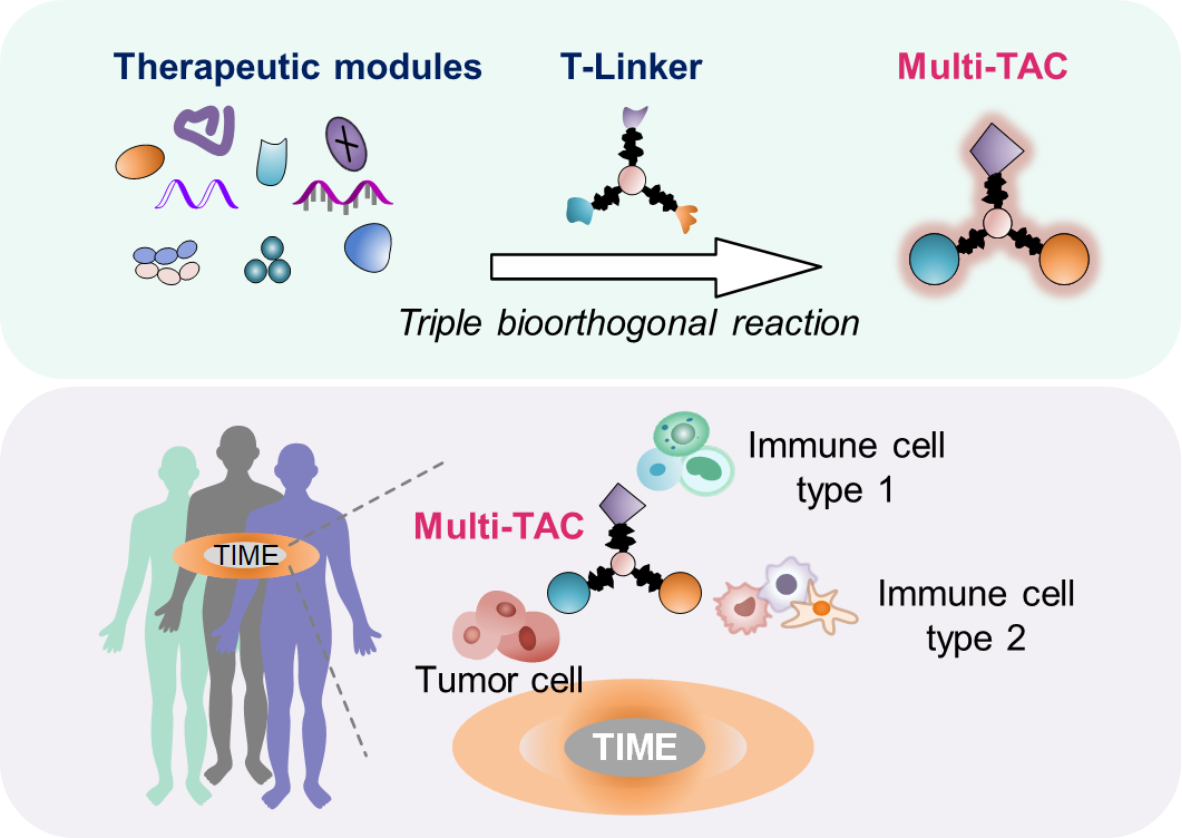
Figure 1 T-Linker-based platform for constructing Multi-TACs targeting TIME
The authors first demonstratedthe development of T-Linker, which featured in triple orthogonal handlesfor precise installation of any three different payloads with virtuallyundetected toxicity in vivo. By leveraging this scaffold, they were able to integrate various therapeutic small molecules and biomolecules as multimodal targeting chimeras (Multi-TACs).
They next systematically studied the EGFR-CD3-PDL1 Multi-TAC, whichco-engaged T-dendritic cells (DCs) inside tumorswith enhanced antitumor immunity as shown in multi-cell co-cultures, three humanized mouse models and 44clinical samples across 6 cancers. To demonstrate the generality of the platform, theyfurther developed EGFR-CD3-CD16 and HER2-CD3-(IMDQ)6Multi-TACs for synergizing T-NK cells and T-myeloid cells respectively.
In summary, a triple orthogonal linker (T-Linker)-basedprogrammable platform has been developed to enable the integration of therapeutic modules intomultimodal targeting chimeras (Multi-TACs), which supports the tumor-targeted co-engagementof different immune cells within thetumor-immune microenvironment.
Associate professor Feng Lin (Peking University) in Peng R. Chen’s lab is the first author.The corresponding author of this work is professor Peng R. Chen, Jianzhong Jeff Xi (Peking University), Xiaozheng Kang (Chinese Academyof Medical Sciences and Peking Union Medical College), Yan Li (Nanjing University) and Jian Lin (Peking University Third Hospital). This work was supported by College of Chemistry and Molecular Engineering at Peking University, National Natural Science Foundation of China, Peking-Tsinghua Center for Life Sciences and XPLORER PRIZE in TencentFoundation.
Link of the paper: https://www.cell.com/cell/abstract/S0092-8674(24)01198-X